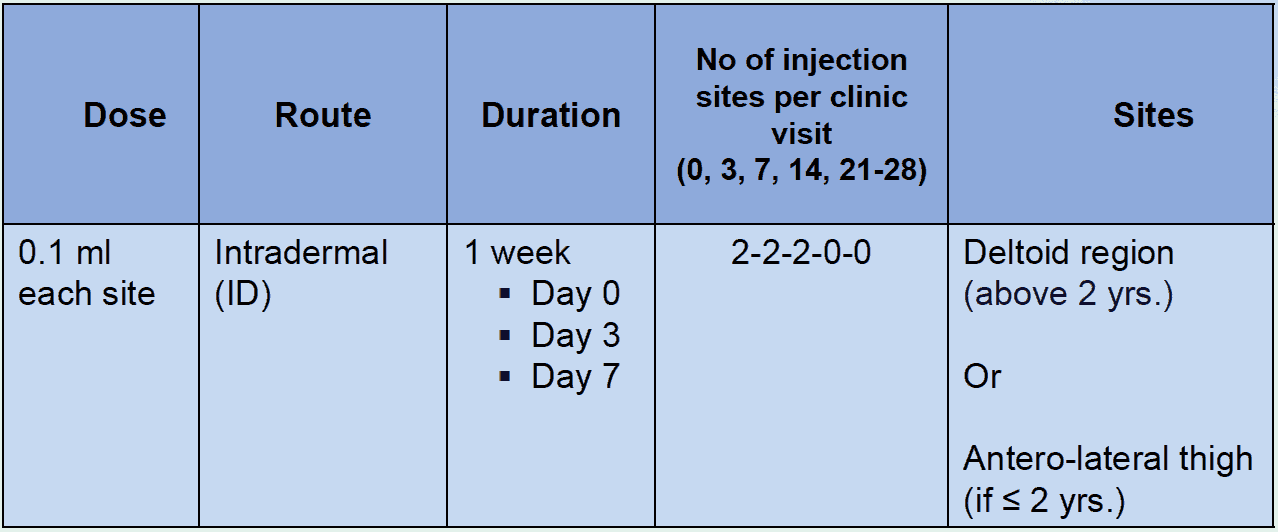
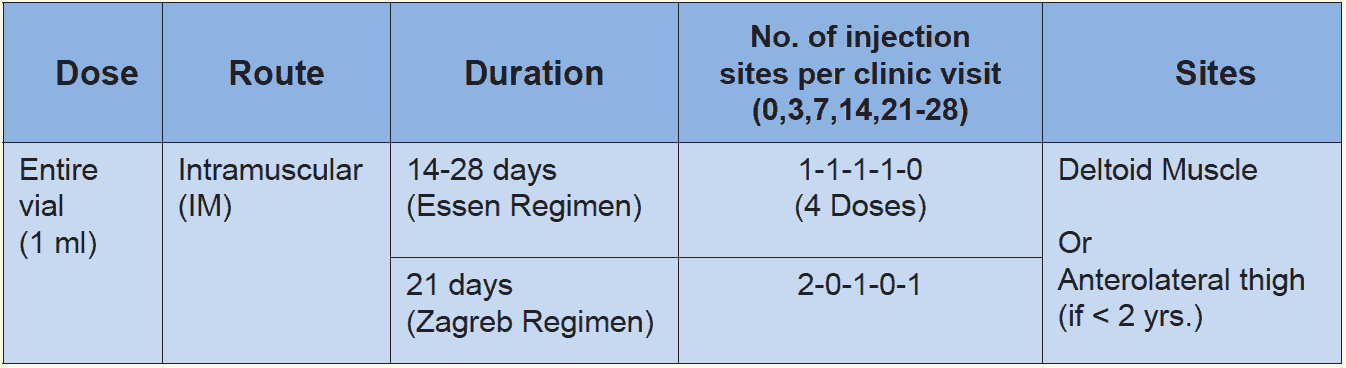
RIG should be administered to all people with category III exposure and those with category II exposure who are immune-compromised. Most of the new equine immunoglobulin (ERIG) preparations are potent, highly purified, safe and considerably less expensive than human rabies immunoglobulin. However, they are of heterologous origin and carry a small risk of an anaphylactic reaction. There are no scientific grounds for performing a skin test before administering equine immunoglobulin because testing does not predict reactions. The treating physician should be prepared to manage anaphylaxis which, although rare, could occur during any stage of administration. The anti-rabies serum/rabies immunoglobulin (RIG) provides passive immunity in the form of a readymade antibodies to tide over the initial phase of the infection.
Indication of RIG:
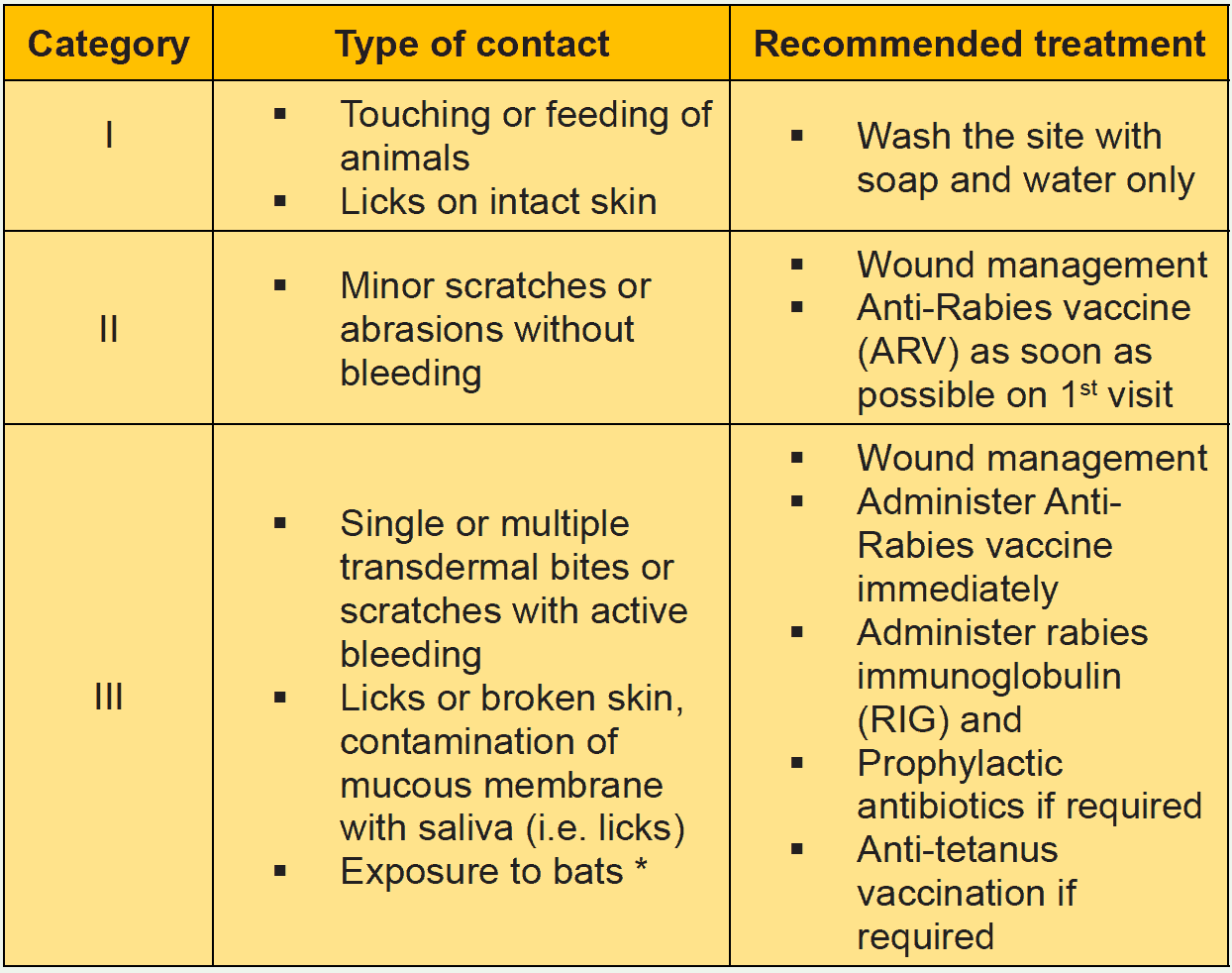
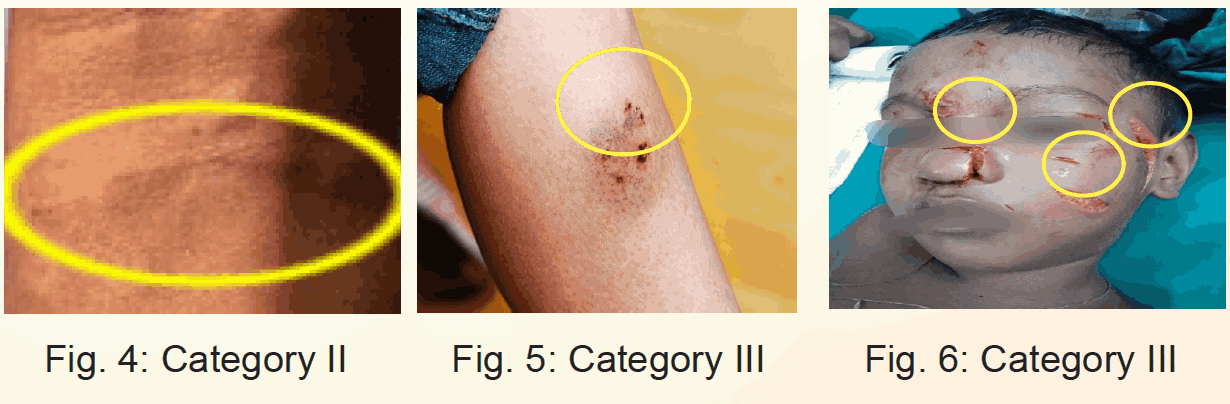
- All WHO-Category III exposures.
- Bites by all wild animals viz. by mongoose, jackal, fox etc.
- Category II exposures in immunocompromised/ immunosuppressed individuals, including HIV infected people & AIDS patients.
Two types of RIGs are available and recommended to use:
i. Equine Rabies Immunoglobulin (ERIG)
ERIG is of heterologous origin raised by hyper-immunization of horses. However, currently manufactured ERIGs are highly purified and the occurrence of adverse events has been significantly reduced.
Dose of rabies ERIG: The dose of equine rabies immunoglobulin (ERIG) is 40 IU per kg body weight the patient (up to a maximum of 3000 IU).
ii. Human Rabies Immunoglobulin (HRIG)
HRIG are free from the side effects encountered in a serum of heterologous origin (i.e. of animal origin), and because of their longer half-life (21 days), are given as dose of 20 IU/kg. HRIG is a homologous biological preparation. HRIG preparation is available in concentration of 150 IU/ml.
Dose of rabies HRIG: The dose of the human rabies immunoglobulin (HRIG) is 20 IU per kg body weight (maximum 1500 IU).
Administration of RIG:
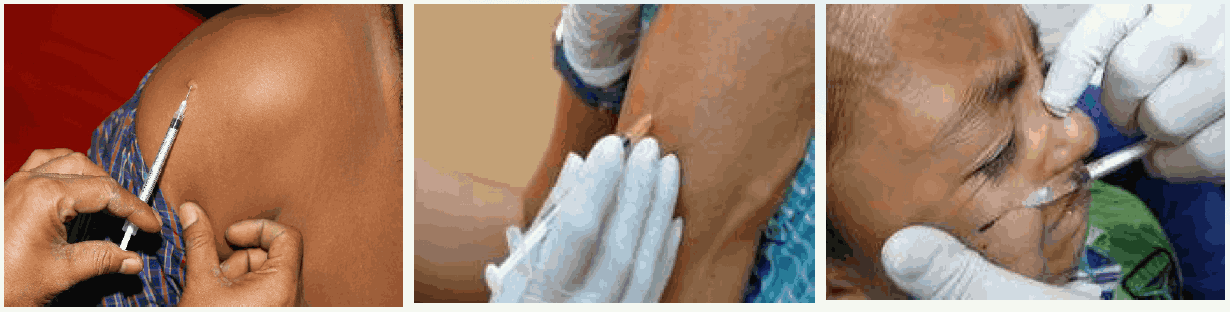
The calculated dose of RIG should be infiltrated as much as possible into the anatomically feasible sites into and around the bite wounds. Multiple needle injections into the wound should be avoided. Any remaining volume of Rabies Immunoglobulin (RIG) should be indicated to rinse the exposure or wound sites. This procedure provides more effective than injecting the remaining Rabies Immunoglobulin (RIG) volume intramuscularly at a distance from the wound or exposure sites. Since RIG may partially suppress the active antibodies, no more than the recommended dose should be given.
Animal bite wounds inflicted can be severe and multiple or, especially in small children. In such cases (WHO Category III), the calculated dose of the rabies immunoglobulin may not be sufficient to infiltrate all wounds. In these circumstances, it is advisable to dilute the RIG in sterile normal saline to be able to permit infiltration of all wounds. RIG should never be administered in the same syringe (ARV & RIG together) or at the same anatomical site as a vaccine.
If RIG was not administered when the anti-Rabies vaccination started, it could be administered until the 7th (seventh) day after administering the first dose of the Anti-Rabies vaccine. Beyond the 7th (seventh) day, RIG is not indicated since an antibody response due to the anti-rabies vaccine is presumed to have occurred. Administration of Rabies Vaccine stimulates the production of neutralizing antibodies by the patient’s immune system. If the patient came several days or months late and the anti-rabies vaccine was not started, then RIG can be used (at bite site) in Category-III. But then attending physician will evaluate each case and do the necessary as required.
The rabies immunoglobulin (ERIG/HRIG) should always be brought to room temperature (20ºC – 25ºC) before use.
Management if anaphylactic reaction occurs:
The patient should be hospitalized for 24-48 hours for observation and management by:
- Adrenaline: The dose is 0.5 ml of 0.1 percent solution (1 in 1000, 1mg/ml) for adults and 0.01 ml/kg body weight for children, injected intramuscularly (IM).
- Inj Hydrocortisone: 100 mg stat.
- Inj. Chlorpheniramine
- Inj. Ranitidine
- Oxygen and others, as necessary management for anaphylaxis/ shock
The patient is sensitive to ERIG, then HRIG should be used (if possible). A patient who had prior exposure to antisera (e.g-Anti-tetanus, serum, antidiphtheria serum) should receive a subcutaneous dose of Inj. Adrenaline (the requirement will be half dose of that required for treatment for anaphylaxis).
Tolerance and Side Effects:
- There may be transient tenderness/redness at the injection site.
- Brief rise in body temperature.
- Skin reactions are infrequent.
- RIG must never be given intravenously since this could produce symptoms of shock, especially in patients with antibody deficiency syndromes.
- Serum sickness may occur in 1% to 6% of patients, usually 7 to 10 days after injection of ERIG.
- Serum sickness is characterized by joint pain, proteinuria etc. It can be managed by non-steroidal anti-inflammatory drugs (NSAID) and H2 blockers.
Precautions to be taken while administering RIGs:
- All emergency drugs and facilities for managing any adverse reactions must be available.
- The RIG vial(s) taken out from the refrigerator should be kept outside for a few minutes before administration to the patient (to warm it to room/body temperature).
- RIG should be administered before starting anti-rabies vaccination.
- RIG should not be administered in the same syringe (ARV & RIG together) as the vaccine or at the same site as a vaccine.
- Pregnancy is not a contra-indication for RIG and anti-rabies vaccination when indicated.
- While infiltrating RIG into bite wounds, care must be taken to avoid injecting blood vessels and nerves. Anatomical feasibility must always be kept in mind while injecting RIG.
- While injecting into fingertips, care must be taken to avoid compartment syndrome.
- In small children with multiple bites, dilute RIG with Normal sterile saline if the volume is insufficient for infiltration in and around all wounds.
- Keep the patient under observation for at least two hours after ERIG administration and send it home.
- The treating physician should be prepared to manage anaphylaxis, which could occur at any stage of administration of ERIG.