Objective of Treatment of Uncomplicated Malaria:
The clinical objectives of treating uncomplicated malaria are to cure the infection as rapidly as possible and to prevent progression to severe disease. "Cure" is defined as elimination of all parasites from the body. The public health objectives of treatment are to prevent onward transmission of the infection to others and to prevent the emergence and spread of resistance to antimalarial drugs.
First line treatment:
Artemether +Lumefantrine combination (ACT) in 6 divided doses over 3 days
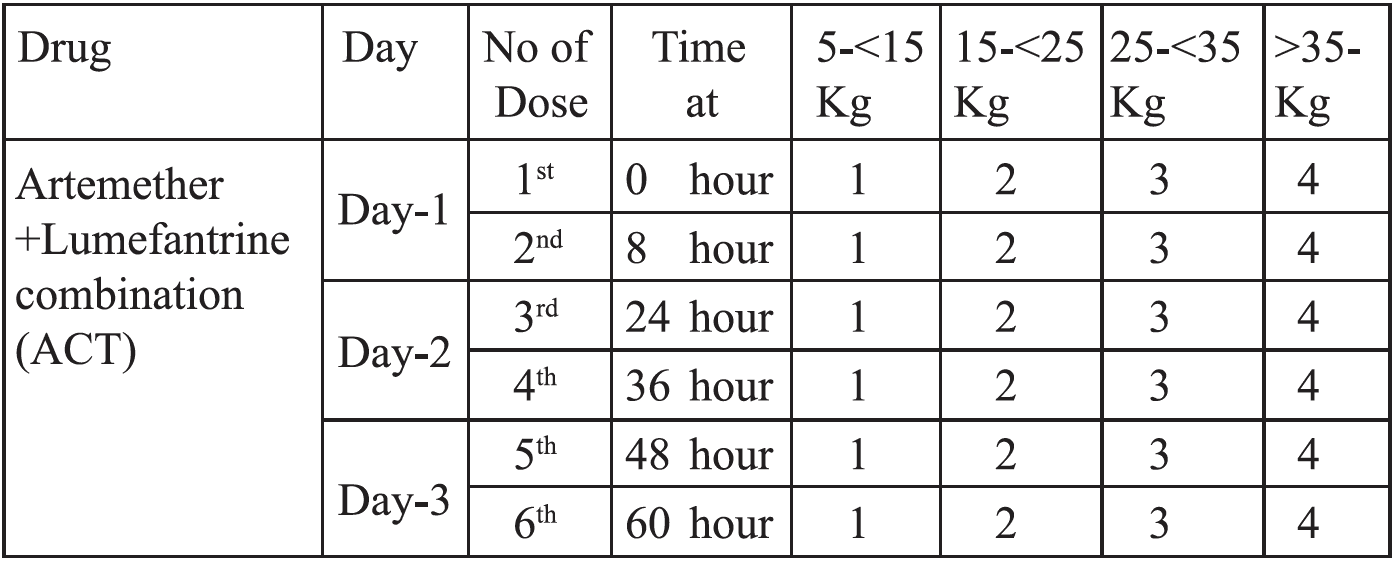
Artemether + Lumefantrine combination (ACT) (20mg + 120 mg) should be started immediately after confirming the diagnosis (0 hours). The second dose should be given 8 hours after the first dose. The subsequent dose will be given 24 hours after first dose or 16 hours after giving second dose. Then the dose are to be given 12 hourly until the total 6 doses have been achieved. The calculated dose for adults and children are given in the box (e.g. for adults 4 tab stat. Second dose is given 8 hours after first dose. Then 4 tab 12 hourly for next two days).
Absorption of lumefantrine is enhanced by co-administration with fat. Patients or caregivers should be informed that this. ACT should be taken immediately after food or a fat containing drink ( e.g. milk), particularly on the second and third days of treatment.
If Artemether +Lumefantrin combination (ACT) cannot be given for any reason then follow alternative treatment.
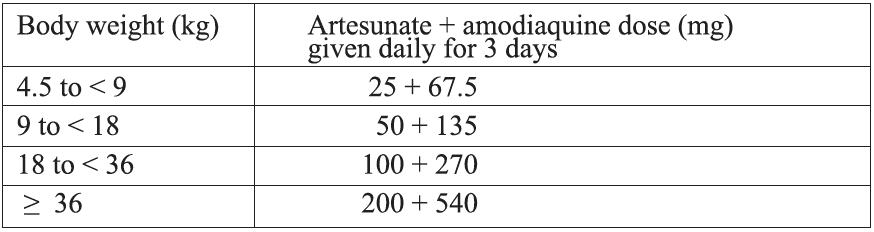
Alternative treatment:i) Artesunate + Amodiaquine*
Formulations currently available: A fixed-dose combination in tablets containing 25 + 67.5 mg, 50 + 135 mg or 100 + 270 mg of artesunate and amodiaquine, respectively.
Target dose and range: The target dose (and range) are 4 (2-10) mg/kg bw/day artesunate and 10 (7 .5-15) mg/kg bw/day amodiaquine once a day for 3 days. A total therapeutic dose range of 6-30 mg/kg kw/day artesunate and 22.5-45 mg/kg bw/dose amodiaquine is recommended.
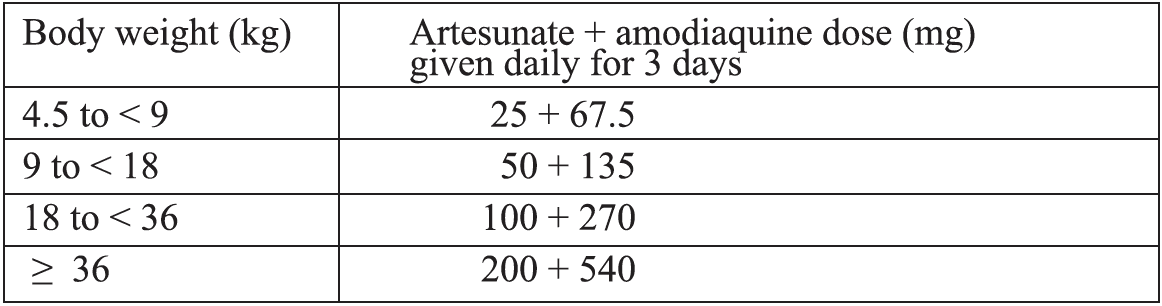
Treatment failure after amodiaquine monotherapy was more frequent among children who were underweight for their age. Therefore, their response to artesunate + amodiaquine treatment should be closely monitored.
• Artesunate + Amodiaquines associated with severe neutropenia, particularly in patients co-infected with HIV and especially in those on zidovudine and/or cotrimoxazole. Concomitant use of efavirenz increases exposure to amodiaquine and hepatotoxicity. Thus, concomitant use of artesunate + amodiaquine by patients taking zidovudine, efavirenz and cotrimoxazole should be avoided, unless this is the only ACT promptly available.
No significant changes in the pharmacokinetics of amodiaquine or its metabolite desethylamodiaquine have been observed during the second and third trimesters of pregnancy; therefore, no dosage adjustments are recommended.
No effect of age has been observed on the plasma concentrations of amodiaquine and desethylamodiaquine, so no dose adjustment by age is indicated. Few data are available on the pharmacokinetics of amodiaquine in the first year of life.
ii) Artesunate + Mefloquine
Formulations currently available: A fixed-dose formulation of paediatric tablets containing 25 mg artesunate and 55 mg mefloquine hydrochloride (equivalent to 50 mg mefloquine base) and adult tablets containing 100 mg artesunate and 220 mg mefloquine hydrochloride (equivalent to 200 mg mefloquine base).
Target dose and range: Target doses (ranges) of 4 (2-10) mg/kg bw/day artestmate and 8.3 (7-11) mg/kg bw/day mefloquine, given once a day for 3 days.
Mefloquine increases incidence of nausea, vomiting, dizziness, dysphoria and sleep disturbance in clinical trials, but these symptoms are seldom debilitating and where this ACT has been used, it has generally been well tolerated.
To reduce acute vomiting and optimize absorption, the total mefloquine dose should preferably be split over 3 days, as in current fixed-dose combinations.
As concomitant use of rifampicin decreases exposure to mefloquine, potentially decreasing its efficacy, patients taking this drug should be followed up carefully to identify treatment failures.
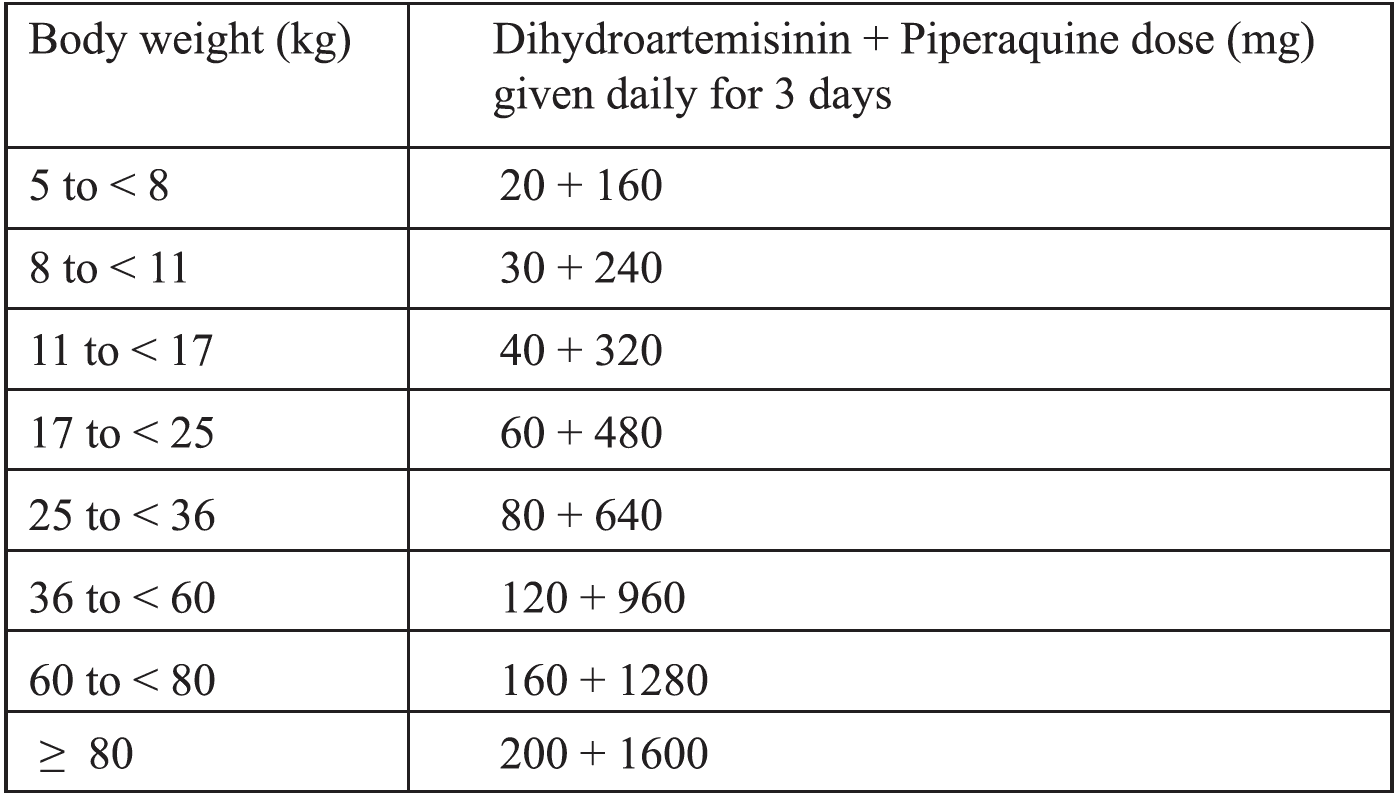
iii) Dihydroartemisinin + Piperaquine
Children weighing <25kg treated with dihydroartemisinin + piperaquine should receive a minimum of2.5 mg/kg bw/day of dihydroartemisinin and 20 mg/kg bw/day of piperaquine daily for 3 days.
Other alternative treatment:
❖ Quinine 7 days+ Tetracycline 7 days (Q7+T7) or
❖ Quinine 7 days+ Doxycycline 7 days (Q7+D7) or
❖ Quinine 7 days + Clindamycin 7 days (Q7+C7)
{Tetracycline and Doxycycline are contraindicated in children younger than 8 years old and in pregnant and lactating women)
Tab Quinine is to be given at a dose of 10 mg/kg body weight 8 hourly for 7 days. The calculated dose for adults and children are given in the box.
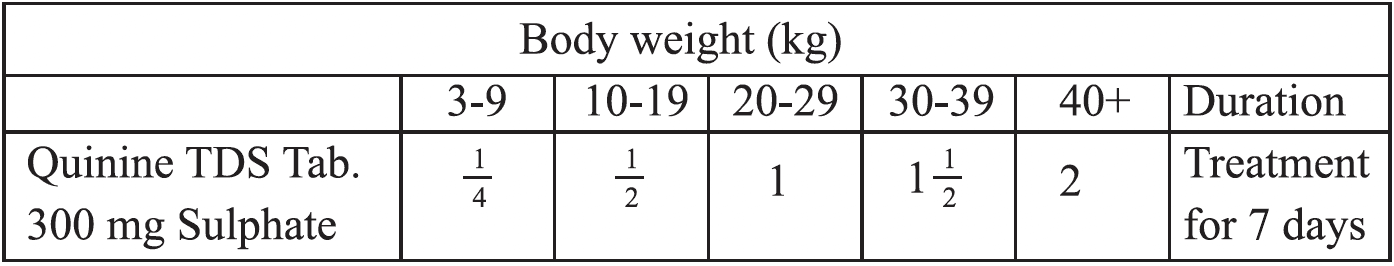
❖ Teteracycline: 250 mg 6 hourly for 7 days
❖ Doxycycline: 100 mg once daily for 7 days
❖ Clindamycin: 10 mg/kg twice daily for 7 days
The Artemether + Lumefantrine (ACT) & Quinine+ Tetracycline/ Doxycycline /Clindamycin can be alternatively used if there is failure of any regimen. So if a patient had received Artemether + Lumefantrine (ACT) and after completion of the course still have uncomplicated malaria (parasitaemia), he or she will be treated with Quinine + Tetracycline/Doxycycline/Clindamycin and if any patient had received Quinine + Tetracycline/Doxycycline/Clindamycin will be treated with ACT.
Reducing the transmissibility of P. falciparum infections:
Primaquine: 0.25 mg/kg single dose to be given on 1st day of ACT or Q7T7 /Q7D7 /Q7C7 treatment
Primaquine should not be given to:
❖ Pregnant women
❖ Infants < 6 months of age and
❖ Lactating women up to 6 months
Treating uncomplicated P. faliparum malaria in special risk groups:
Infants less than 5kg body weight-
Treat infants weighing <5 kg with uncomplicated P. falciparum malaria with an ACT at the same mg/kg bw target dose as for children weighing 5 kg.
Patients co-infected with HIV-
people who have HIV/ AIDS and uncomplicated P falciparum malaria do not use artesunate + amodiaquine if they are also receiving efavirenz or zidovudine.
Non-immune travelers-
Treat travellers with uncomplicated P. falciparum malaria returning to nonendemic area with an ACT.
Uncomplicated hyperparasitaemia-
Persons with P. falciparum hyperparasitaemia ( 4 to 10 % ) are at increased risk of treatment failure, severe malaria and death. They should receive 1st dose of ACT and immediately admitted in the nearest hospital for close monitoring and treatment.
Special issues:
Plasmodium knowlesi: Human infections with the monkey malaria parasite P. knowlesi are being reported from the forested regions of South-East Asia. No P. knowlesi has been reported from Bangladesh.
