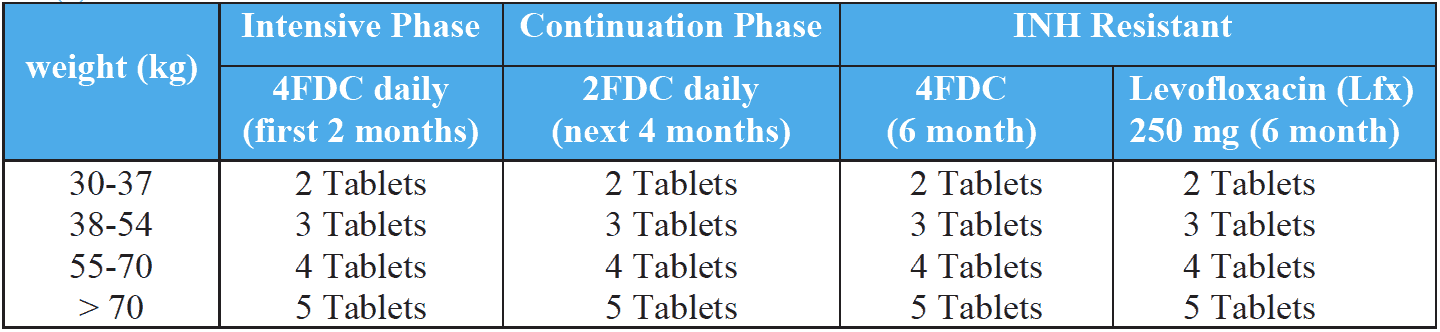
Treatment of drug-susceptible tuberculosis comprises of two phases:
- The intensive phase (IP): This is administered daily for the initial two months (4FDC) of treatment. The objective of combining four drugs in the intensive phase (IP) is to achieve rapid killing of actively multiplying bacillary population. This phase will eliminate naturally occurring drug resistant mutants and prevent the further emergence of drug resistant mutants. The infectious patients quickly become non-infectious (within approximately two weeks of treatment initiation).
- The continuation phase (CP): This is administered for four months (2FDC) and is essential to eliminate the remaining bacterial population (mainly persisters) which are largely responsible for relapses. In some special cases, the CP can be extended beyond 4 months (described above). The drugs are administered daily for the rest of the treatment duration according to the category.
Previously treated (PT) patients, eligible for retreatment, should be referred for a rapid molecular test or drug susceptibility testing to determine the resistance status to at least rifampicin, and also preferably isoniazid.
If R is sensitive but resistance to H is detected (Hr-TB)/ H DST unknown, then the patient is initiated on a 6-month regimen of 5 drugs [6 (H) REZ- Lfx].
If rifampicin resistance is detected, an MDR-TB regimen should be prescribed according to recent drug resistant TB treatment guidelines.
Standardized treatment regimen for each diagnostic category (adults)
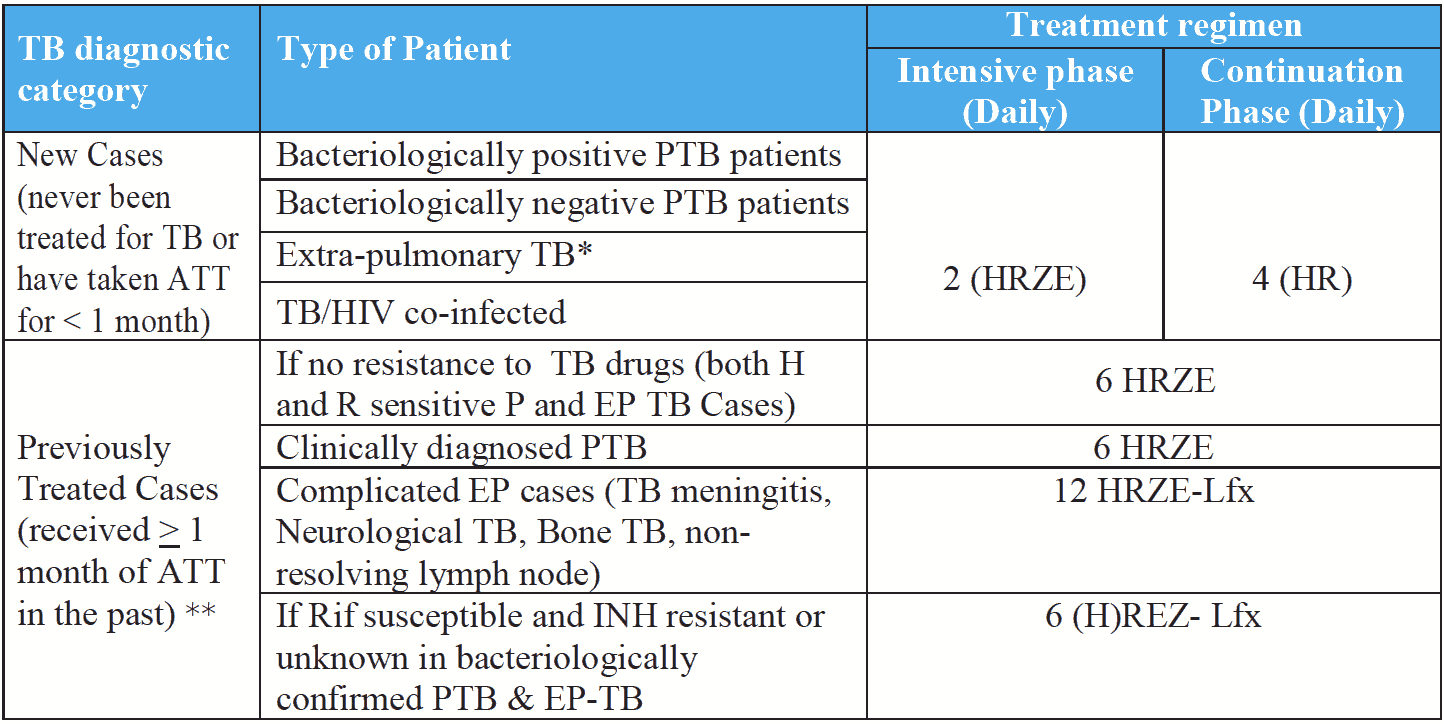
* Treatment for certain EP TB may be prolonged till 12 months if non-resolving lymph nodes at 6 months; 12 months in case of CNS, TB meningitis, bone TB etc.