5.7 Monitoring of Treatment
exp date isn't null, but text field is
Monitoring of tuberculosis patients on treatment include:
- Bacteriological monitoring of pulmonary TB cases by examination of sputum smears at regular intervals during the course of treatment.
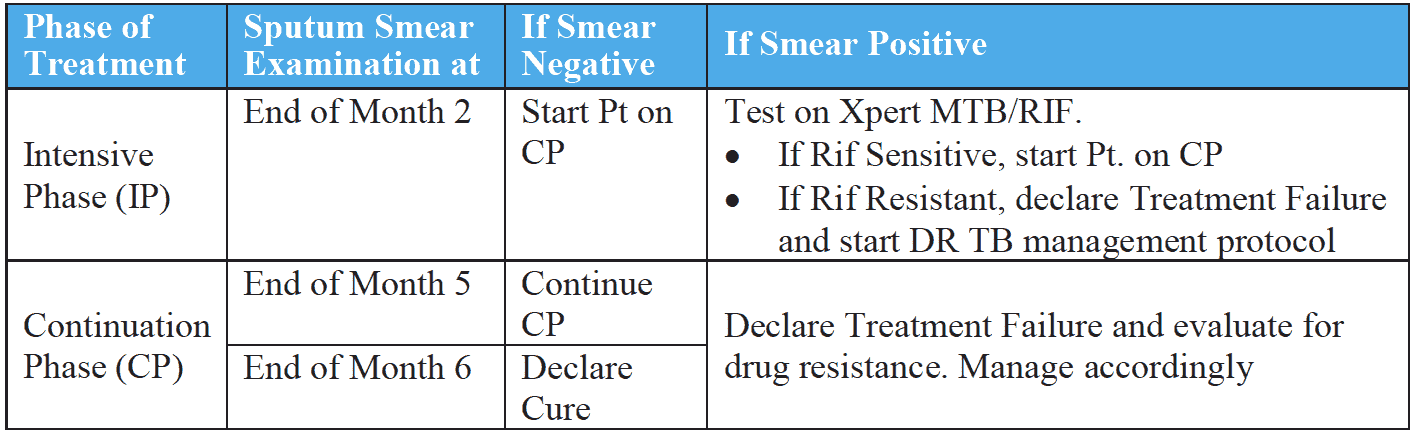
Sputum must be examined at periodic intervals to monitor the progress of treatment for all pulmonary TB patients. The sputum smear examinations are carried out at the end of 2nd month (end of IP), the end of the 5th month and at the end of treatment (6th month). Sputum must also be examined for patients who were smear-negative to start with at the end of the 2nd month of treatment.
If an EP case on treatment develops chest symptoms, then his or her sputum should be collected and examined by smear microscopy/gene Xpert.
In case sputum is positive on smear microscopy in any of the follow up examination, additional samples needs to be collected and sent for Xpert MTB/RIF testing.
Patients on treatment, who remain smear positive at the end of IP (2nd month) but whose Xpert MTB/RIF results are sensitive for rifampicin, should be initiated on the continuation phase of treatment.
- Clinical monitoring is carried out at periodic intervals by checking for symptomatic improvement and weight gain, especially in the case of extra-pulmonary and clinically diagnosed pulmonary TB cases.
The patient’s weight should be monitored on a monthly basis and the drug dosages should be modified if it crosses the initial weight band. Details of all medications given, bacteriological response and adverse reactions should be documented on the patient’s records, including the TB Treatment Card.
Adherence to treatment needs to be monitored by reviewing the treatment cards for drug intake during intensive phase and drug collection during the continuation phase and whenever possible by interviewing the patients.
* If a patient is found to harbor a drug-resistant strain of TB at any time during therapy, treatment is declared as failed and the patient should be referred for DR-TB management.
* For new pulmonary TB patients being treated with a Rifampicin containing regimen, extension of IP is not needed.
