Abbreviation and Acronyms
exp date isn't null, but text field is
|
ACSM |
Advocacy, Communication, and Social Mobilization |
|
ACF |
Active Case Finding |
|
ACH |
Air Changes per Hour |
|
ADR |
Adverse Drug Reaction |
|
AFB |
Acid-fast bacillus |
|
AIC |
Airborne Infection Control |
|
AIDS |
Acquired Immune Deficiency Syndrome |
|
AMC |
Annual Maintenance Contract |
|
AMK |
Amikacin |
|
ART |
Antiretroviral Therapy |
|
ATT |
Anti-tubercular Treatment |
|
BCAP |
Bedaquiline Conditional Access Programme |
|
BCG |
Bacille Calmette-Guerin |
|
BDQ |
Bedaquiline |
|
BMI |
Body Mass Index |
|
BSC |
Biological Safety Cabinet |
|
C&DST |
Culture and Drug Susceptibility Testing |
|
CB-NAAT |
Cartridge-based Nucleic Acid Amplification Test |
|
CHW |
Community Health Worker |
|
CFZ |
Clofazimine |
|
CI |
Contact Investigation |
|
CME |
Continuing Medical Education |
|
CPT |
Cotrimoxazole Preventive Therapy |
|
CSO |
Civil Society Organization |
|
CXR |
Chest X-Ray |
|
DLM |
Delamanid |
|
DM |
Diabetes Mellitus |
|
DOT |
Directly Observed Therapy |
|
DOTS |
Directly Observed Therapy, Short-course |
|
DR-TB |
Drug Resistant Tuberculosis |
|
DRS |
Drug Resistance Surveillance |
|
DST |
Drug Susceptibility Testing |
|
DS-TB |
Drug-Sensitive Tuberculosis |
|
DQA |
Data Quality Assurance |
|
E |
Ethambutol |
|
EPTB |
Extrapulmonary Tuberculosis |
|
EQA |
External Quality Assurance |
|
FDC |
Fixed-dose Combination |
|
FLD |
First-line Drugs |
|
FQ |
Fluoroquinolone |
|
GDF |
Global Drug Facility |
|
GFATM |
Global Fund to Fight HIV/AIDS, Tuberculosis and Malaria |
|
H |
Isoniazid |
|
HA |
Health Assistant |
|
HBC |
High Burden Countries |
|
HCW |
Health Care Worker |
|
HIV |
Human Immunodeficiency Virus |
|
HR |
Human Resources |
|
HS |
Health Systems |
|
HSS |
Health Systems Strengthening |
|
IC |
Infection Control |
|
ICF |
Intensified Case Finding |
|
ICT |
Information and Communication Technologies |
|
IEC |
Information, Education, and Communication |
|
IGRA |
Interferon-Gamma Release Assay |
|
INH |
Isoniazid |
|
IPC |
Infection Prevention and Control |
|
JMM |
Joint Monitoring Mission |
|
KPI |
Key Performance Indicator |
|
LFX |
Levofloxacin |
|
LPA |
Line Probe Assay |
|
LT |
Laboratory Technician |
|
LTBI |
Latent Tuberculosis Infection |
|
LTFU |
Lost To Follow Up |
|
LZD |
Linezolid |
|
M&E |
Monitoring and Evaluation |
|
MDG |
Millennium Development Goal |
|
MDR |
Multidrug Resistance |
|
MDR-TB |
Multidrug Resistant Tuberculosis |
|
MFX |
Moxifloxacin |
|
MGIT |
Mycobacteria Growth Indicator Tube |
|
MTB |
Mycobacterium Tuberculosis |
|
NCD |
Noncommunicable Disease |
|
NGO |
Non-governmental Organization |
|
NGS |
Next-Generation Sequencing |
|
NTRL |
National TB Reference Laboratory |
|
NSP |
National Strategic Plan |
|
NTM |
Non-Tuberculous Mycobacteria |
|
NTP |
National Tuberculosis Control Programme |
|
OR |
Operational Research |
|
PCR |
Polymerase Chain Reaction |
|
PICT |
Provider Initiated Counseling and Testing |
|
PLHIV |
People Living with HIV/AIDS |
|
PP |
Private Practitioner |
|
PPM |
Public-Private Mix |
|
PTB |
Pulmonary Tuberculosis |
|
QA |
Quality Assurance |
|
R / RIF |
Rifampicin |
|
RR-TB |
Rifampicin-Resistant Tuberculosis |
|
RTRL |
Regional TB Reference Laboratory |
|
SDG |
Sustainable Development Goals |
|
SEARO |
South East Asia Regional Office |
|
SLD |
Second-line Drugs |
|
SL-DST |
Second-line Drug Susceptibility Testing |
|
SLI |
Second-line Injectable Drugs |
|
SNRL |
Supra National Reference Laboratory |
|
TA |
Technical Assistance |
|
TAT |
Turnaround Times |
|
TALF |
Treatment After Lost to Follow Up |
|
TB |
Tuberculosis |
|
TLCA |
TB and Leprosy Control Assistant |
|
TOR |
Terms of Reference |
|
TPT |
TB Preventive Treatment |
|
TST |
Tuberculin Skin Test |
|
UDST |
Universal Drug Susceptibility Tests |
|
UN |
United Nations |
|
UNHLM |
United Nations High-level Meeting |
|
UVGI |
Ultraviolet Germicidal Irradiation |
|
WHO |
World Health Organization |
|
WRD |
WHO Recommended Rapid Diagnostics |
|
XDR-TB |
Extensively Drug Resistant Tuberculosis |
|
Z |
Pyrazinamide |
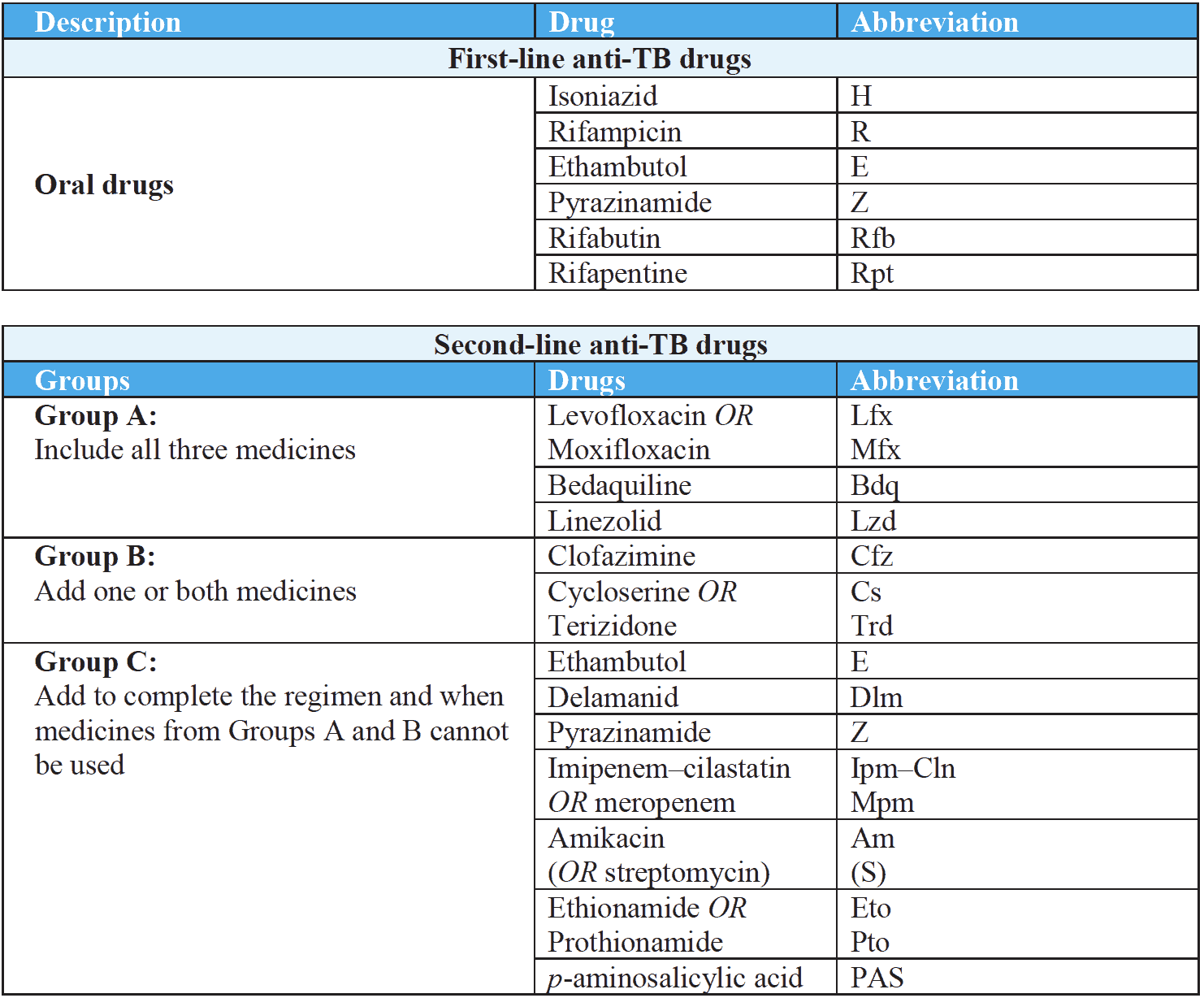
Anti-Tubercular Drugs Classification